Home » Lyme Disease
Lyme disease is a tick-borne bacterial infection that is caused by a spirochete bacterium called Borrelia. If Lyme is left untreated, it can cause chronic joint inflammation, neurological disorders, and cognitive defects. According to the Centers for Disease Control and Prevention (CDC), approximately 30,000 cases of Lyme disease are reported in the United States each year. However, due to poor diagnostic testing, the CDC estimates the actual number is closer to 476,000.1
Lyme disease is the most common vector-borne disease in the United States and Europe. Cases of Lyme disease are most commonly reported in the Northeast, upper Midwest, and Northwestern states, but cases have been reported in nearly all states in the United States. In Europe, the highest incidence of Lyme is reported in Austria, the Czech Republic, Germany, Slovenia, and other northern countries bordering the Baltic Sea.2
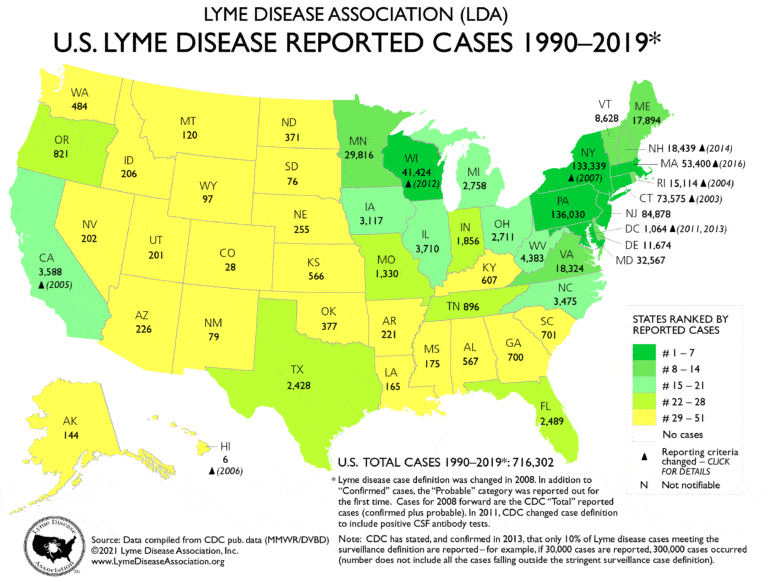
Image Source Lyme Disease Association
The CDC currently recommends a two-tiered approach when testing for Lyme disease.
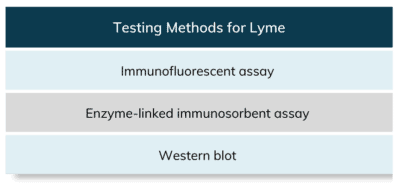
There are several direct and indirect testing methods for detecting Lyme.
Lyme disease is difficult to diagnose because common symptoms such as headache, dizziness, and joint or body pain are also common in many other diseases. Another sign indicative of Lyme disease is the presence of erythema migrans (EM) a circular red rash at the site of the tick bite, which only appears in a quarter of people that have been infected with Lyme bacteria.3
Current diagnostic tests for Lyme have certain limitations. Approximately 3.4 million tests are performed for Lyme disease each year, including serology tests, polymerase chain reaction (PCR) techniques, Western Blot, and blood culture.4 These tests are labor-intensive, can take weeks to process and are subject to high false-negative rates due to their inability to detect the presence of Borrelia. Because of the challenges surrounding diagnostic testing for Lyme disease, patients are frequently misdiagnosed or face a testing odyssey, taking years to reach a correct diagnosis. The limitations of current Lyme disease tests are reflected in the CDC recommendation that the diagnosis remains a clinical one, based on a patient’s symptoms and history, with additional tests only used to provide supporting evidence for the diagnosis.
For patients who are infected with Lyme-causing bacteria, early diagnosis and appropriate treatment can prevent or reduce these complications and the significant associated costs. Preclinical data4 suggests that the T2Lyme™ Panel provides greater accuracy than other diagnostics for identifying Borrelia infections for patients suspected of having early-stage Lyme disease.
The T2Lyme Panel is a direct-from-blood test for the detection of active Lyme disease and detects the species Borrelia afzelii, Borrelia burgdorferi, and Borrelia garinii that cause Lyme disease across the globe. The Panel is designed to provide greater accuracy in the diagnosis of early Lyme disease, which may help improve care and lead to better patient outcomes. T2Lyme runs on the FDA-cleared T2Dx® Instrument, the same instrument currently used to run the FDA-cleared T2Bacteria® and T2Candida® Panels. The T2Lyme Panel was granted Breakthrough Device Designation by the United States Food and Drug Administration (FDA) on July 11, 2022. This designation brings T2 Biosystems one step closer to providing clinicians with a valuable tool to detect Lyme disease earlier.
The T2Lyme Panel is not currently FDA-cleared or commercially available.
For more information on the T2Lyme Panel, please contact us today.
……………………………………….
T2 Biosystems, an emerging leader in the field of in vitro diagnostics, is dedicated to saving lives and reducing the cost of healthcare by empowering clinicians to effectively treat patients faster than ever before. T2 Biosystems is focused on addressing critical unmet needs in healthcare starting with sepsis, one of the deadliest and most expensive conditions in hospitals today.
The T2Dx Instrument, the T2Bacteria and T2Candida Panels have received marketing authorization from the U.S. Food and Drug Administration. All other T2 Biosystems products are considered investigational and for research use only.
T2 Biosystems®, T2MR®, T2Bacteria®, T2Candida®, T2Resistance® and T2Dx® are registered trademarks of T2 Biosystems, Inc. “T2Biosystems” and the T2 Biosystems, Inc. logo design are registered trademarks or trademarks of T2Biosystems, Inc. All software and documentation is subject to T2 Biosystems, Inc. copyrights. All rights reserved. T2Direct Diagnostics™, T2HemoStat™, T2Plex™, T2Cauris™, T2Lyme™ and T2SARS-CoV-2™ are trademarks of T2 Biosystems, Inc.
© 2024 T2 Biosystems, Inc.
Please tell us where you are visiting from: United States | Outside the United States