Home » Products & Technology » T2Bacteria Panel
The T2Bacteria® Panel is the first and only FDA-cleared and CE-marked panel to detect six clinically relevant bacterial pathogens in 3 to 5 hours, directly from a whole blood sample. E. faecium, S. aureus, K. pneumoniae, A. baumannii, P. aeruginosa, and E. coli represent the majority of bacteremia seen in the emergency department1 and those causing hospital-acquired infections2. In addition, several of these species are known as ESKAPE pathogens3, characterized as virulent and difficult to treat with empiric antimicrobial therapy, making faster species identification and targeted treatment critical to improving patient outcomes2.
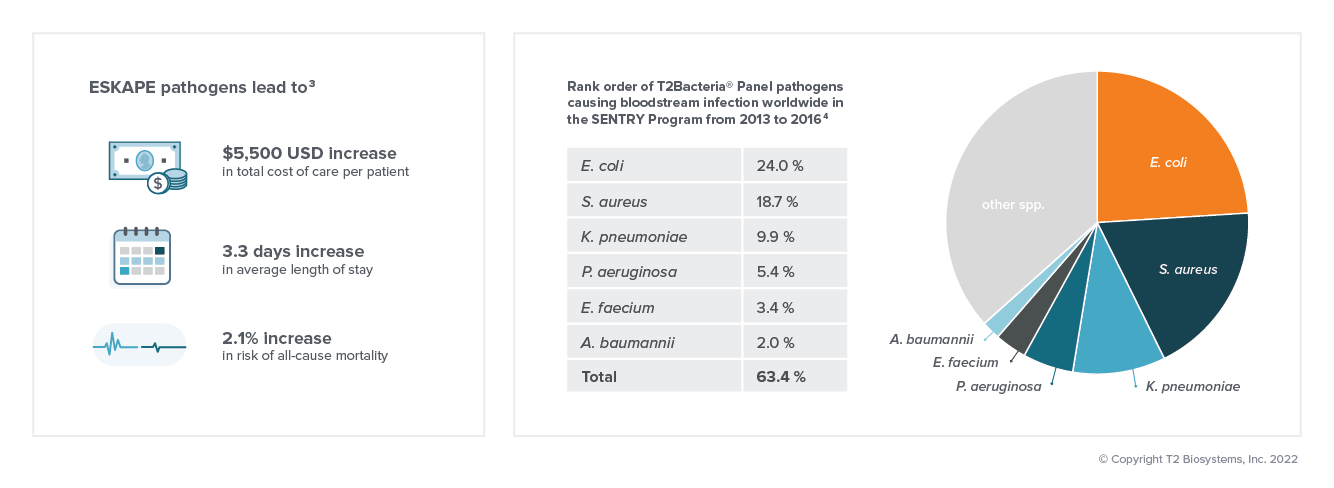
Bacterial bloodstream infections may lead to sepsis. In the absence of species-specific diagnostic information, clinical treatment of sepsis begins with the initiation of empiric therapy. Despite the broad coverage of empiric therapy, it may not adequately address all pathogens, with studies demonstrating that 1 in 5 patients with bloodstream infections receive discordant treatment, which is associated with increased odds of mortality.5 Fast, targeted antimicrobial therapy is especially important for patients in septic shock, whose mortality risk increases by 8% for every hour of delayed appropriate treatment.6
Target therapy sooner by reducing the time to species ID7
Increase clinician confidence in treatment with accurate and reliable results6
Impact patient outcomes and reduce length of stay for patients with bloodstream infections8
Improve antimicrobial stewardship
Real-world clinical case studies have proven the clinical utility of both positive and negative results using the T2Bacteria Panel. Read our case studies to learn more.
……………………………………….

Sensitivity: 90%7
Specificity: 98%7
E. faecium
S. aureus
K. pneumoniae
A. baumannii
P. aeruginosa
E. coli
T2 Biosystems, an emerging leader in the field of in vitro diagnostics, is dedicated to saving lives and reducing the cost of healthcare by empowering clinicians to effectively treat patients faster than ever before. T2 Biosystems is focused on addressing critical unmet needs in healthcare starting with sepsis, one of the deadliest and most expensive conditions in hospitals today.
The T2Dx Instrument, the T2Bacteria and T2Candida Panels have received marketing authorization from the U.S. Food and Drug Administration. All other T2 Biosystems products are considered investigational and for research use only.
T2 Biosystems®, T2MR®, T2Bacteria®, T2Candida®, T2Resistance® and T2Dx® are registered trademarks of T2 Biosystems, Inc. “T2Biosystems” and the T2 Biosystems, Inc. logo design are registered trademarks or trademarks of T2Biosystems, Inc. All software and documentation is subject to T2 Biosystems, Inc. copyrights. All rights reserved. T2Direct Diagnostics™, T2HemoStat™, T2Plex™, T2Cauris™, T2Lyme™ and T2SARS-CoV-2™ are trademarks of T2 Biosystems, Inc.
© 2024 T2 Biosystems, Inc.
Please tell us where you are visiting from: United States | Outside the United States