Home » Products & Technology International » Product Pipeline International
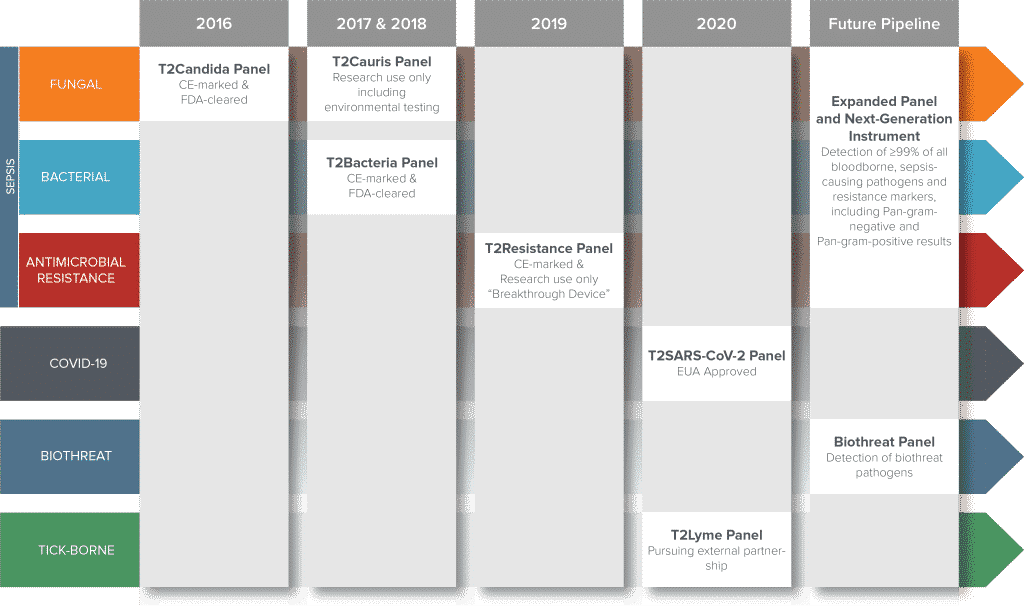
T2 Biosystems has multiple diagnostic applications in development, all designed to enable further breakthroughs in rapid and accurate therapy for patients. The diagnostics are focused on critical, unmet needs in healthcare, where solutions can serve a dual role of improving patient care while reducing costs.
T2Resistance® Panel: T2 Biosystems has partnered with Allergan and CARB-X to develop panels with additional bacterial species and resistance markers. The T2Resistance Panel detects gram-negative and gram-positive resistance markers and is currently available for research use only (RUO) in the U.S. and CE marked on November 20, 2019.
T2Cauris™ Panel RUO: Direct detection of the emerging superbug Candida auris in patient skin and blood samples and hospital environmental samples are now available for research use only (RUO) assays. The CDC* validated the T2Cauris Panel RUO swab test on patient skin samples and published their 2017 findings in Mycoses.1
……………………………………….
1. Sexton DJ, et al. Mycoses, 2018
*The 2017 CDC findings identified the T2Cauris Panel RUO as a rapid diagnostic that demonstrated significant time advantages (<5 hours) compared to culture methods that take 14 days. Additional data from a collaboration with a European hospital was presented by T2 Biosystems in a poster on the T2Cauris Panel at IDWeek 2017.
Learn more about how you can develop additional breakthrough applications utilizing our T2MR technology.
T2 Biosystems, an emerging leader in the field of in vitro diagnostics, is dedicated to saving lives and reducing the cost of healthcare by empowering clinicians to effectively treat patients faster than ever before. T2 Biosystems is focused on addressing critical unmet needs in healthcare starting with sepsis, one of the deadliest and most expensive conditions in hospitals today.
The T2Dx Instrument, the T2Bacteria and T2Candida Panels have received marketing authorization from the U.S. Food and Drug Administration. All other T2 Biosystems products are considered investigational and for research use only.
T2 Biosystems®, T2MR®, T2Bacteria®, T2Candida®, T2Resistance® and T2Dx® are registered trademarks of T2 Biosystems, Inc. “T2Biosystems” and the T2 Biosystems, Inc. logo design are registered trademarks or trademarks of T2Biosystems, Inc. All software and documentation is subject to T2 Biosystems, Inc. copyrights. All rights reserved. T2Direct Diagnostics™, T2HemoStat™, T2Plex™, T2Cauris™, T2Lyme™ and T2SARS-CoV-2™ are trademarks of T2 Biosystems, Inc.
© 2024 T2 Biosystems, Inc.
Please tell us where you are visiting from: United States | Outside the United States